"Pinched" Neurites, and the Infamous Row 4 Cell
Wasn’t sure where to post this, or even if it deserves it’s own topic…I’ll let the community decide.
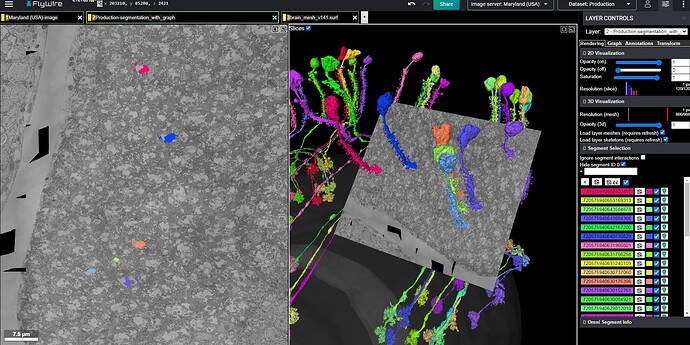
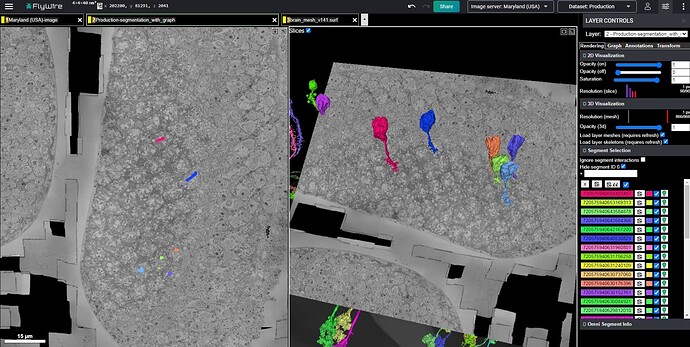
It seems to me that there is a pattern to some of the problems showing up, in the Q&A log. Many of the cells that I have seen there, including the infamous “Row 4 Cell” involve a neurite that suddenly just ends. The pattern I am seeing is that most of them are type L cells, and they tend to suddenly end in the area known as the chiasm (i.e. the “Black Spill” area, discussed in a separate topic), which is essentially the boundary or transition from the medulla to the lamina. As has been documented in the FlyWire blog, and elsewhere, the lamina is essentially a series of modular, repeated structures, known as “Laminar Cartridges” or “Columns”, in which the same cell types repeat the same pattern of structure, over and over (about 800 times, if I’m not mistaken).
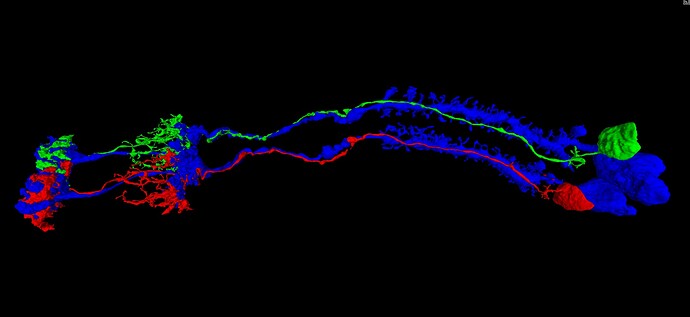
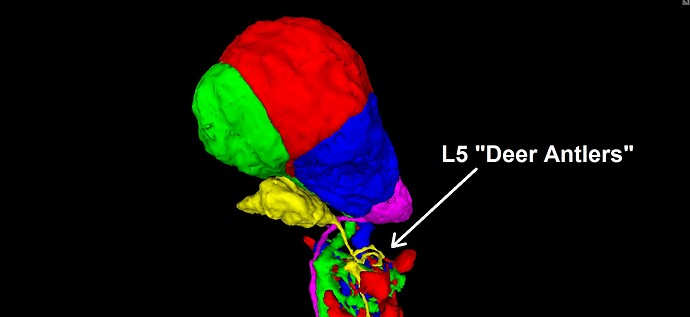
Given that these repeated laminar structures show the same basic arrangement of the component cells, each time, it seems that we can make use of that regularity, in predicting the continuation of the dead-end neurites mentioned above. Applying this approach to the infamous “Row 4 Cell” (which shows up again and again, in rows 84, 121, 123, etc.), yields the solution pictured and linked below. In the view below, the row 4 cell is shown in red, next to a nearby cell of the same type (L5), from an adjacent laminar cartridge/column, shown in green. For reference, the L1 cells of both cartridges/columns are shown in blue. As can be seen, the similarity between the two cartridges/columns is sufficient, in my opinion, to suggest a practical solution strategy. This practical similarity can be further reinforced by comparing with several other nearby cartridges/columns, and I encourage others to do so (I’ve spent days now, looking through the dataset and comparing these structures).
Also, it is worth noting that, although these “pinched” neurites do appear to be actually separated/broken/pinched apart, in the 2D EM image data, the data would look the same if they suddenly reduced in diameter to the point that they passed between slices (i.e. not actually broken, just very thin). Either way, it is a problem that seems to come up over and over again, in the same area, regardless of what the actual explanation may be.
If anyone has any thoughts or insight on this, I would appreciate feedback. I have tried applying this approach on several of the cells in the Q&A log, and the results have been very encouraging…enough so that I felt it deserved mention here, and possibly it’s own topic for discussion.
Cheers, all.
https://ngl.flywire.ai/?json_url=https://globalv1.flywire-daf.com/nglstate/5950851256418304